 The Ask:
The Ask:
A global medical devices company wanted to embark on the implementation of the EU MDR.
Scale:
- 250+ Technical Files
- Seven global locations
Results and Benefits
- Project teams traceable to the EU MDR 2017/745 – MDR “To Do” list
- Easy, live tracking and management of the Global EU MDR program
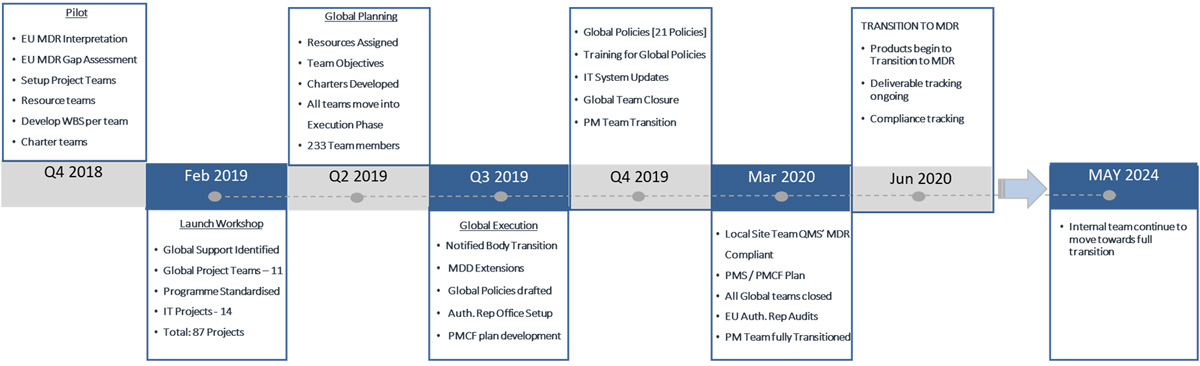
- Global EU MDR program successfully moved to stable execution
- 87 Project teams initiated, planned, and moved into successful execution
- 233 Project team members engaged and mobilised
- Standardised toolkit utilised & knowledge sharing leading to reduced planning phase
- Global governance structure & communication plan Implemented
- Graphical toolkit utilised to facilitate remote & group collaboration
- 8 Months – Start to execution phase for 87 project teams
Challenges:
- Very little internal experience in large scale programme management
- The organisation did not have the experience to bring a global programme of this scale through the required phases and into execution
- ‘No internal’ toolkit to address a programme of this scale
- To move a program of this scale required a distinct set of tools that were standardised, scalable and transparent
- How to measure & track progress over the programme lifecycle? (4 Years)
- Overloaded Internal Staff: How can we make this management as hands-free as possible?
Implication
- EU MDR will be legally binding
- Products not compliant to EU MDR will no longer be saleable in the European Union
- Annual sales in excess of $300 Million at risk
- Significant hit to the global organisation bottom line
Objective
- Traceability from the project team scope to the EU MDR Regulation
- Governance model & program structure standardised & scalable across seven global sites
- Reduce the management burden of the program as internal staff are heavily loaded
- Capability for live accurate transparent measurement of progress
- Bring the program to stable execution before transition
- Transition: Hand over the management of the program to the internal team
The Approach:
Toolkit
- Explic8 EU MDR Gap Assessment Template
- Explic8 Work Breakdown Structure Template
- Explic8 EU MDR Status Report
- Explic8 EU MDR Progress & Compliance Tracking Database

